Home
Tutorial's
Image collection
Calculator
Projects
MCQ's
3d Models
Invention Hub
How it works
Download Our app
Module 22: Incandescent Lamp
When the appliances are connected to an electric supply, a large amount of heat is produced due to the high-resistance coil. The coil has the ability to melt with a high resistance point; this heating effect of electric current is utilized by many electrical heating appliances.
Construction:
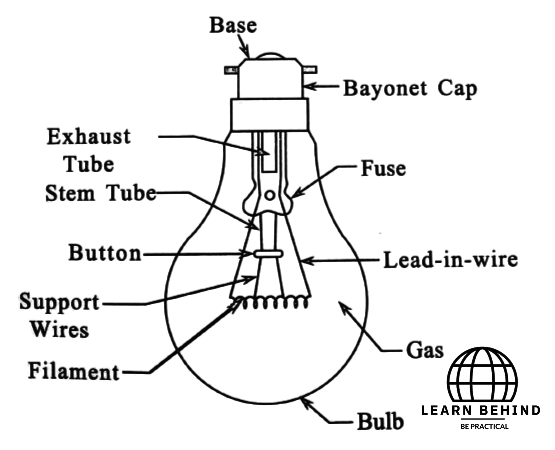
Basically, an incandescent lamp is made of a thin filament of tungsten enclosed by a bulb of glass. This filament is held up by thin wires and heated by electric current so as to yield light. The bulb is normally filled with inert gas to protect the filament from oxidation; argon or nitrogen can be used.. In its base, electrical connections and mechanical support are realized. While the design of an incandescent lamp is simple, it is relatively inefficient. Most energy ended up being converted into heat rather than light, which means that they are slowly being replaced by more efficient options like LED and fluorescent lamps.
what is oxidation and why oxidation effects inside filament?
Oxidation is the process of losing electrons by a substance. The filament, usually made of tungsten, in an incandescent bulb is very easily oxidized in the presence of oxygen.
These very high temperatures, developed by the electric current passing through the filament, increase the oxidation process. When tungsten reacts with oxygen, it becomes tungsten oxide, a very brittle compound. Ultimately, the formation of these oxides makes the filament structure weak and thin and thus easily breakable. For this reason, in many cases, incandescent lamps are enclosed with some inert gas like argon or nitrogen that blocks the access of oxygen to the filament, therefore not allowing oxidation to occur.
Basically, oxidation is the nemesis of the filament, which will cause it to degrade, hence shortening the life expectancy of an incandescent lamp.
Working:
This would be so because an incandescent lamp works on the principle of incandescence, which is its ability to produce light through heat.
This is due to the fact that, as the electric current passes through the thin tungsten filament in the middle of the glass bulb, it suffers resistance. This resistance heats the filament to squanderingly high temperatures. As the filament becomes hot, it starts to glow, emitting visible light.
The gas enclosed is typically inert, e.g., argon or nitrogen, so as to stop oxidation on the filament, which may cause it to burn out. This helps in reducing the rate of the oxidation of the filament, hence increasing its life span.
Although making incandescent lamps is easy and cheap, they are relatively ineffective as a great part of the energy is converted into heat but not into light. The lamps are inefficient, and this resulted in a constant replacement by other alternatives such as LED and fluorescent lamps.
Why We Use Inert Gases in Incandescent Lamps
Oxidation prevention: Since the filament works under extremely high temperatures, it would easily react with oxygen to form tungsten oxide. This oxide would be very brittle and hence weaken the filament, thus usually causing breakage. Inert gases are chemically unreactive and hence prevent this oxidation process.
Reducing Evaporation: At very high temperatures, tungsten atoms evaporate from the filament; hence, its thickness reduces and eventually breaks. Inert gases slow down this evaporation process, therefore increasing the life of the bulb.
Improved Thermal Conductivity: While this is not the major reason, some of the inert gases, such as argon, have lower thermal conductivity compared to air. Thus, it reduces the loss of heat from the filament and, for that matter, may improve efficiency.
Related articles
Related Calculator
- Heat Calculator
- Voltage Calculator
- Current Calculator
- Series Capacitor Calculators
- Parallel Capacitor Calculator
Related Inventions
